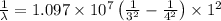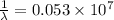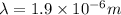Chemistry, 25.04.2020 23:12, connorvoss5805
Consider a Hydrogen atom in the 3rd excited state (n = 4). The maximum wavelength of light that can be emitted is
9.7 x 10^-8 m
1.9 x 10^-6 m
1.03 x 10^-7 m
2.5 x 10^-5 m

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, Ezekielcassese
What is the percentage composition of carbon in the compound ch4
Answers: 1


Chemistry, 22.06.2019 18:30, tanviknawale
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1

Chemistry, 22.06.2019 23:00, tovarclaudia055
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1
Do you know the correct answer?
Consider a Hydrogen atom in the 3rd excited state (n = 4). The maximum wavelength of light that can...
Questions in other subjects:

Physics, 18.11.2020 22:20

Health, 18.11.2020 22:20

Social Studies, 18.11.2020 22:20

Chemistry, 18.11.2020 22:20




Health, 18.11.2020 22:20



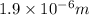
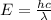
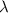 = Wavelength of radiation
= Wavelength of radiation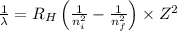
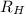 = Rydberg's Constant =
= Rydberg's Constant =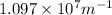
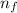 = Higher energy level = 3
= Higher energy level = 3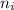 = Lower energy level = 4
= Lower energy level = 4