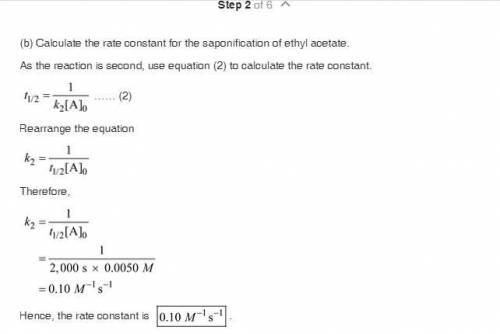
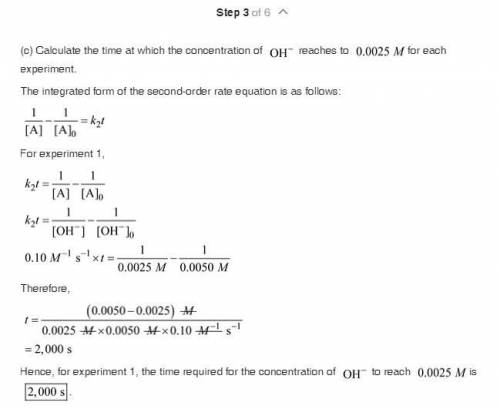
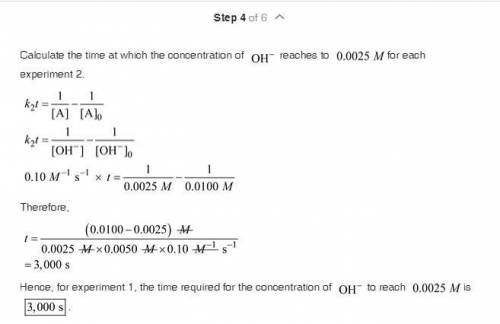
The saponification (hydrolysis) of ethyl acetate occurs according to the stoichiometric relation CH 3 COOC 2 H 5 + OH - S CH 3 COO - + C 2 H 5 OH. The reaction can be followed by monitoring the disappearance of OH - . The following experimental results were obtained at 25 °C: Initial concentration of OH " (M) Initial concentration of CH 3 COOC 2 H 5 (M) Half-life, t ½ , s 0.0050 0.0050 2000 0.0100 0.0100 1000 There is no significant dependence on the concentrations of products of the reaction. a. What is the overall kinetic order of the reaction? b. Calculate a value for the rate coefficient, including appropriate units. c. How long would it take for the concentration of OH - to reach 0.0025 M for each experiment? d. Based on your answer to part (a), what are possible rate laws for this reaction? e. Carefully describe a single experiment that would enable you to decide which of the possibilities of part (d) is most nearly correct.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:40, kellypechacekoyc1b3
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 19:40, powberier6979
What is the wavelength of a 3*10^12 hz infrared wave a 3*10^20m b 1* 10^4m c 3*10^-3m d 1*10^-4 m
Answers: 1

Chemistry, 22.06.2019 22:00, genyjoannerubiera
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1

Do you know the correct answer?
The saponification (hydrolysis) of ethyl acetate occurs according to the stoichiometric relation CH...
Questions in other subjects:

Biology, 30.05.2020 05:59

Mathematics, 30.05.2020 05:59




Spanish, 30.05.2020 05:59

Mathematics, 30.05.2020 05:59


Mathematics, 30.05.2020 05:59

Mathematics, 30.05.2020 05:59


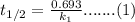
![t = \frac{1}{k_2 [A]_0} .........(2)](/tpl/images/0626/6042/d1ea9.png)