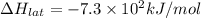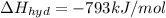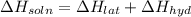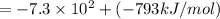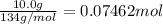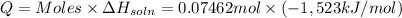Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:30, Apple557
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1


Chemistry, 22.06.2019 20:30, kittybatch345
Is a chemical message sent by another individual.
Answers: 1
Do you know the correct answer?
Lithium iodide has a lattice energy of −7.3×102kj/mol and a heat of hydration of −793kj/mol. find th...
Questions in other subjects:


Mathematics, 30.10.2020 20:10


Mathematics, 30.10.2020 20:10

Chemistry, 30.10.2020 20:10


Mathematics, 30.10.2020 20:10

Computers and Technology, 30.10.2020 20:10


Mathematics, 30.10.2020 20:10