
Chemistry, 25.04.2020 03:00, destinywashere101
A chemist titrates 216.8 mL of a 0.3200 M butanoic acid (HC3H7CO2) solution with a 0.3200 M solution of NaOH. The titration setup shows that the butanoic acid solution is in the (Erlenmeyer) flask, and the NaOH solution is in the burette. The pKa of butanoic acid is 4.82. Calculate the pH of the solution in the flask after the chemist has added 231.4 mL of NaOH solution to it.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, vanessa051266
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1


Chemistry, 22.06.2019 16:30, Eddie997
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 22.06.2019 18:30, tanviknawale
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1
Do you know the correct answer?
A chemist titrates 216.8 mL of a 0.3200 M butanoic acid (HC3H7CO2) solution with a 0.3200 M solution...
Questions in other subjects:











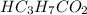 = 0.32 M,
= 0.32 M,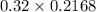
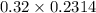
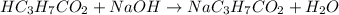
![[OH^{-}] = \frac{n}{V}](/tpl/images/0626/3976/691f7.png)
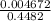
![-log [OH^{-}]](/tpl/images/0626/3976/337a4.png)




