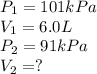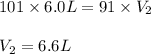In one city, a balloon with a volume of 6.0 L is filled with air at 101 kPa
pressure. The ball...

Chemistry, 25.04.2020 01:40, deaishaajennings123
In one city, a balloon with a volume of 6.0 L is filled with air at 101 kPa
pressure. The balloon is then taken to a second city at a much higher
altitude. At this second city, atmospheric pressure is only 91 kPa. If the
temperature is the same in both places, what will be the new volume of the
balloon?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, dpchill5232
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 16:00, winnie45
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2

Do you know the correct answer?
Questions in other subjects:


Arts, 29.08.2019 03:00

Mathematics, 29.08.2019 03:00



Health, 29.08.2019 03:00


Health, 29.08.2019 03:00

Mathematics, 29.08.2019 03:00

History, 29.08.2019 03:00


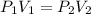
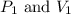 are initial pressure and volume.
are initial pressure and volume.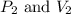 are final pressure and volume.
are final pressure and volume.