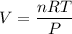Chemistry, 24.04.2020 23:21, reearamrup27
Calculate how many liters of H2 gas at STP are produced from 2.11 moles of aluminum metal.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:30, claudr03
If we compare and contrast electromagnetic waves with sound waves, all but one statement is true. that is a) sound waves require a medium to travel while electromagnetic waves do not. b) electromagnetic waves can travel through the vacuum of space while sound waves cannot. c) electromagnetic waves must have a medium in which to travel, but sound waves can travel anywhere. eliminate d) sound waves must bounce off of matter in order to travel while electromagnetic waves do not require matter to be present.
Answers: 3

Chemistry, 22.06.2019 11:50, hamidaakter936848
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 23.06.2019 03:00, yoongislaugh
What volume does 1.70 ×10–3 mol of chlorine gas occupy if its temperature is 20.2 °c and its pressure is 795 mm hg?
Answers: 3
Do you know the correct answer?
Calculate how many liters of H2 gas at STP are produced from 2.11 moles of aluminum metal....
Questions in other subjects:

Mathematics, 25.08.2019 23:20

Mathematics, 25.08.2019 23:20


Mathematics, 25.08.2019 23:20


History, 25.08.2019 23:20


Chemistry, 25.08.2019 23:20