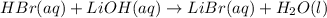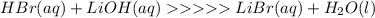Chemistry, 08.12.2019 22:31, ambarpena14
Write the balanced chemical equation for the neutralization reaction that occurs when an aqueous solution of hydrobromic acid (hbr) is mixed with an aqueous solution of lithium hydroxide (lioh).
indicate the physical states of the reactants and products using the abbreviations (s), (l), (g), or (aq) for solids, liquids, gases, or aqueous solutions, respectively.
express your answer as a chemical equation.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, jonloya264
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 14:30, emilymartinez75
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1


Chemistry, 23.06.2019 00:30, danielmartinez024m
Maya wrote if you step to describe how carbon circulates between the atmosphere and living organisms
Answers: 1
Do you know the correct answer?
Write the balanced chemical equation for the neutralization reaction that occurs when an aqueous sol...
Questions in other subjects:



Biology, 19.08.2019 09:30


English, 19.08.2019 09:30


Mathematics, 19.08.2019 09:30


English, 19.08.2019 09:30