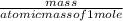Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, medlinalex
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 22:30, skinniestoflegends
Consider a culture medium on which only gram-positive organisms such as staphylococcus aureus colonies can grow due to an elevated nacl level. a yellow halo surrounds the growth, indicating the bacterium fermented a sugar in the medium, decreasing the ph as a result and changing the color of a ph indicator chemical. this type of medium would be referred to as a differential and enrichment culture.
Answers: 2

Chemistry, 23.06.2019 03:00, duplessistoccara
Abaker touches a pie right after taking it out of the oven. which statement best explains why the pie feels hot?
Answers: 2

Chemistry, 23.06.2019 09:00, floressavanna15
What properties would have caused early researchers to name hydrogen "inflammable air”
Answers: 3
Do you know the correct answer?
Si + 2Cl2 + SiC14
What mass of SiCl4 is formed when 10.0 grams of Si and 60.0
grams of C...
What mass of SiCl4 is formed when 10.0 grams of Si and 60.0
grams of C...
Questions in other subjects:




Chemistry, 29.06.2019 15:40





Chemistry, 29.06.2019 15:40

Chemistry, 29.06.2019 15:40


 = 169.8 grams/mole
= 169.8 grams/mole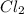 ⇒ Si
⇒ Si