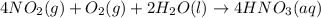Chemistry, 27.10.2019 14:43, kharmaculpepper
Nitric acid in acid rain forms when gaseous nitrogen dioxide pollutant reacts with gaseous oxygen and liquid water to form aqueous nitric acid. write a balanced chemical equation for this reaction. express your answer as a chemical equation. identify all of the phases in your answer.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 05:50, kawaunmartinjr10
Aseismic wave is energy released as the result of rock movement along a fault. t or f ?
Answers: 1


Chemistry, 23.06.2019 07:20, msladycie8831
F1.5 mol of nabh4 react, how many moles of b2h6 are formed? 2 nabh4(aq) + h2so4(aq) → 2 h2(g) + na2so4(aq) + b2h6(g)
Answers: 1

Chemistry, 23.06.2019 09:20, weridness80
Which of the following occurs along coasts during the day?
Answers: 3
Do you know the correct answer?
Nitric acid in acid rain forms when gaseous nitrogen dioxide pollutant reacts with gaseous oxygen an...
Questions in other subjects:


Mathematics, 22.04.2020 23:05

Social Studies, 22.04.2020 23:05

Biology, 22.04.2020 23:05