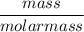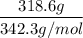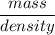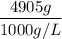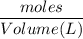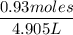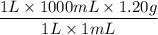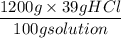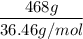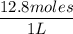A solution is prepared by dissolving 318.6 g sucrose (C12H22O11) in 4905 g of water. Determine the molarity of the solution
Commercial grade HCl solutions are typically 39.0% (by mass) HCl in water. Determine the molarity of the HCl, if the solution has a density of 1.20 g/mL.
Commercial grade HCl solutions are typically 39.0% (by mass) HCl in water. Determine the mol of the HCl, if the solution has a density of 1.20 g/mL.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, ronny80
Supongamos que estás estudiando dos estrellas. ambas estrellas tienen la misma magnitud aparente, pero la estrella a tiene una magnitud absoluta mayor que la estrella b. ¿que puedes decir acerca de la distancia a la tierra de estas dos estrellas?
Answers: 3

Chemistry, 22.06.2019 07:20, mathman783
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1

Chemistry, 22.06.2019 09:30, kevinh2683
Apump contains 0.5 l of air at 203 kpa. you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2
Do you know the correct answer?
A solution is prepared by dissolving 318.6 g sucrose (C12H22O11) in 4905 g of water. Determine the m...
Questions in other subjects:

Mathematics, 19.12.2020 02:20



Social Studies, 19.12.2020 02:20

History, 19.12.2020 02:20



Mathematics, 19.12.2020 02:20

Mathematics, 19.12.2020 02:20