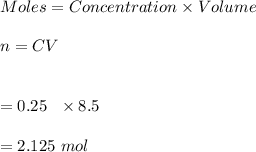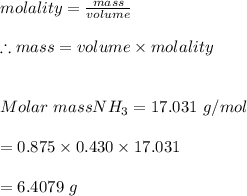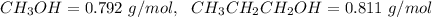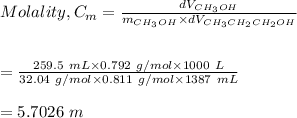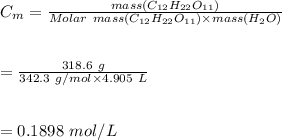Chemistry, 24.04.2020 01:00, robert7248
1. Calculate the number of moles of sulfuric acid that is contained in 250 mL of 8.500 M sulfuric acid solution
2. 7.300 moles of sodium nitrite are needed for a reaction. The solution is 5.450 M. How many mL are needed?
3. What mass (in g) of NH3 must be dissolved in 875 g of methanol to make a 0.430 molal solution?
4. Calculate the molality of a solution that is prepared by mixing 259.5 mL of CH3OH
(d = 0.792 g/mL) and 1387 mL of CH3CH2CH2OH (d = 0.811 g/mL)
5. A solution is prepared by dissolving 318.6 g sucrose (C12H22O11) in 4905 g of water. Determine the molarity of the solution

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 12:30, fvmousdiana
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3
Do you know the correct answer?
1. Calculate the number of moles of sulfuric acid that is contained in 250 mL of 8.500 M sulfuric ac...
Questions in other subjects:


Mathematics, 21.09.2019 16:00


Mathematics, 21.09.2019 16:00



Mathematics, 21.09.2019 16:00