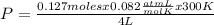Chemistry, 23.04.2020 23:47, isabel81ie
5.6 g of solid CO2 is put in an empty sealed 4.00 L container at a temperature of
300 K. When all the solid CO2 becomes gas, what will be the pressure in the
container? *
34.5 atm
O
O
0.78 atm
0.006 atrh
None of the other answers

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 21:20, 50057543
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2

Chemistry, 22.06.2019 23:00, tovarclaudia055
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1

Chemistry, 23.06.2019 01:30, joyelewis58
Will a solution form when the solvent and solute are both nonpolar? a. not likely b. never c. most likely
Answers: 1
Do you know the correct answer?
5.6 g of solid CO2 is put in an empty sealed 4.00 L container at a temperature of
300 K. When...
300 K. When...
Questions in other subjects:


Mathematics, 20.04.2021 18:50

Mathematics, 20.04.2021 18:50


History, 20.04.2021 18:50

Social Studies, 20.04.2021 18:50

Mathematics, 20.04.2021 18:50

Mathematics, 20.04.2021 18:50



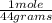 = 0.127 moles (being 44
= 0.127 moles (being 44 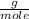 the molar mass of CO₂, that is, the amount of mass that a substance contains in one mole.)R= 0.082
the molar mass of CO₂, that is, the amount of mass that a substance contains in one mole.)R= 0.082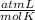 T= 300 K
T= 300 K