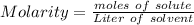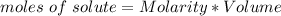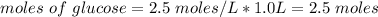Chemistry, 01.01.2020 18:31, vinniemccray70
How many moles of glucose are present in 1.0 liters of a 2.5 m solution of glucose water

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, alexandroperez13
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3


Chemistry, 22.06.2019 03:10, lilque6112
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3

Chemistry, 22.06.2019 05:00, adrian128383
What forms when chemical reactions combine pollution with sunlight?
Answers: 1
Do you know the correct answer?
How many moles of glucose are present in 1.0 liters of a 2.5 m solution of glucose water...
Questions in other subjects:

Mathematics, 02.09.2019 04:10


Biology, 02.09.2019 04:10

English, 02.09.2019 04:10


Social Studies, 02.09.2019 04:10



Mathematics, 02.09.2019 04:10