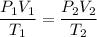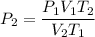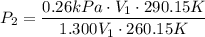Chemistry, 23.04.2020 22:23, christiantorres57
For many purposes we can treat methane (CH) as an ideal gas at temperatures above its boiling point of -161. °C. Suppose the temperature of a sample of methane gas is raised from -13.0 °C to 17.0°C, and at the same time the pressure is changed. If the initial pressure was 0.26 kPa and the volume increased by 30.0%, what is the final pressure? Round your answer to the correct number of significant digits.

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 03:50, daniel9299
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3
Do you know the correct answer?
For many purposes we can treat methane (CH) as an ideal gas at temperatures above its boiling point...
Questions in other subjects:

English, 05.10.2020 23:01

History, 05.10.2020 23:01

Chemistry, 05.10.2020 23:01



English, 05.10.2020 23:01