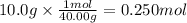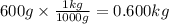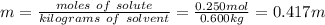Calculate the molality of a solution prepared by dissolving 10.0 g of NaCl in 600g of
water....

Chemistry, 23.04.2020 21:29, cordovatierra16
Calculate the molality of a solution prepared by dissolving 10.0 g of NaCl in 600g of
water.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, huangjianhe135
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 23.06.2019 04:10, nabeelunique
An unknown substance has been shown to have metallic bonds. which of the following is most likely a property of this substance? a. low conductivity b. low boiling point c. high malleability d. high solubility in water
Answers: 2
Do you know the correct answer?
Questions in other subjects:


Mathematics, 08.01.2021 04:50

Mathematics, 08.01.2021 04:50



Spanish, 08.01.2021 04:50

Mathematics, 08.01.2021 04:50

Health, 08.01.2021 04:50

Mathematics, 08.01.2021 04:50