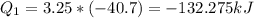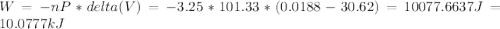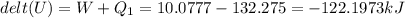Chemistry, 23.04.2020 20:22, vlarocca17
The vaporization of 1 mole of liquid water (system) at 100.9 C, 1.00 atm, is endothermic.
H2O(L) + 40.7 Kj > H2O (g).
Assume at exactly 100.0°c and 1.00 atm total pressure, 1.00 mole of liquid water and 1.00 mole of water vapor occupy 18.80 ml and 30.62 l, respectively.
The calculated work done on or by the system when 3.25 mol of liquid water vaporizes is -10077J.
Calculate the water's change in internal energy.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, queenkimm26
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 06:00, Sarahinator04
0.09 moles of sodium sulfate in 12 ml of solution
Answers: 3

Chemistry, 22.06.2019 12:30, pup88
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2

Chemistry, 22.06.2019 15:20, merrickrittany
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1
Do you know the correct answer?
The vaporization of 1 mole of liquid water (system) at 100.9 C, 1.00 atm, is endothermic.
H2O...
H2O...
Questions in other subjects:

English, 01.07.2019 17:00

Mathematics, 01.07.2019 17:00


Mathematics, 01.07.2019 17:00

Social Studies, 01.07.2019 17:00



Business, 01.07.2019 17:00


Mathematics, 01.07.2019 17:00