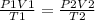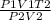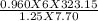Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:40, georgehall3027
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2



Chemistry, 23.06.2019 01:00, daniel1480
Which fossil fuel is mainly used for heating and cooking? a. electricity b. coal c. petroleum d. natural gas
Answers: 2
Do you know the correct answer?
A sample of krypton has a volume of 6.00 L, and the pressure is 0.960 atm. If the final temperature...
Questions in other subjects:






Mathematics, 13.02.2021 01:00