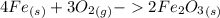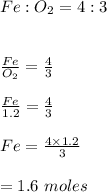Chemistry, 23.04.2020 05:20, tiffanydowell13
A chemistry student was asked to calculate the number of moles of iron required to react with 1.20 mol of oxygen to produce iron(III) oxide. His calculation is shown below: 1.20

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 21.06.2019 19:00, anonymous176
Iknow the answer to 13 is b and 14 is d. i just need to know why the correct answers are correct
Answers: 3
Do you know the correct answer?
A chemistry student was asked to calculate the number of moles of iron required to react with 1.20 m...
Questions in other subjects:

English, 25.01.2021 04:40


English, 25.01.2021 04:40


Mathematics, 25.01.2021 04:40

Mathematics, 25.01.2021 04:40

English, 25.01.2021 04:40


Geography, 25.01.2021 04:40