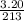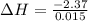Chemistry, 23.04.2020 02:56, LindaCat78
A "coffee-cup" calorimetry experiment is run for the dissolution of 3.20 g of aluminum nitrate placed into 103.2 mL of water. The temperature of the solution is initially at 23.2 oC. After the reaction takes place, the temperature of the solution is 17.7 oC. How much heat was absorbed or lost by the surroundings? Use 4.184 J/goC for the specific heat of the solution. Put your answer in units of kJ and make sure the sign is correct. What would be the enthalpy for the dissolution reaction of one mole of aluminum nitrate? Put your answer in kJ/mol and watch the sign for the enthalpy.

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 12:00, ctyrector
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1

Chemistry, 22.06.2019 20:20, carcon2019
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3
Do you know the correct answer?
A "coffee-cup" calorimetry experiment is run for the dissolution of 3.20 g of aluminum nitrate place...
Questions in other subjects:


Mathematics, 01.07.2020 22:01


Mathematics, 01.07.2020 22:01




English, 01.07.2020 22:01



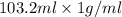
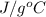 .
.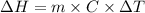
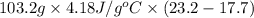
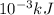 . So, 2372.5 J will be converted into kJ as follows.
. So, 2372.5 J will be converted into kJ as follows.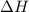 = 2.37 kJ
= 2.37 kJ 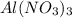 = 213 g/mol
= 213 g/mol