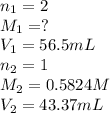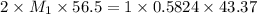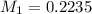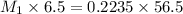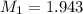A titration is performed to determine the amount of sulfuric acid, H2SO4, in a 6.5 mL sample taken from car battery. About 50 mL of water is added to the sample, and then it is titrated with 43.37 mL of a standard 0.5824 molar NaOH solution. You balanced this reaction in a previous problem. How what is the molar concentration of sulfuric acid in the original sample

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, ashleyjaslin
Calculate the expected ph values of the buffer systems from the experiments (a, b,c, d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 04:30, logan12345677885675
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2

Chemistry, 22.06.2019 05:30, nuclearfire278
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease. correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1

Chemistry, 22.06.2019 17:00, destinyycooper
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2
Do you know the correct answer?
A titration is performed to determine the amount of sulfuric acid, H2SO4, in a 6.5 mL sample taken f...
Questions in other subjects:









Mathematics, 23.07.2019 08:30


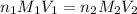
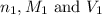 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 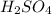
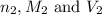 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.