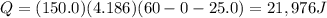CaO(s) + H2O(l) - Ca(OH)2(s)
enthalpy of rxn= -63.7 kJ/molrxn
Calcium oxide, CaO(...

Chemistry, 22.04.2020 23:16, ethangorrell67
CaO(s) + H2O(l) - Ca(OH)2(s)
enthalpy of rxn= -63.7 kJ/molrxn
Calcium oxide, CaO(s), has been proposed as a substance that can be used to heat water quickly for portable heating packs or for cooking. When placed in water, Cao(s) reacts as shown by the equation above.
A student wants to design a heating pad that could heat a 150.0 g sample of water from 25.0°C to 60.0°C.
Calculate the amount of heat, in joules, that the water must absorb for its
temperature to change by this amount. (Assume that the specific heat capacity
of the water is 4.18 J/gK).

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, kdenormandie3122
Geothermal energy for industrial use is available almost anywhere. a. true b. false
Answers: 2

Chemistry, 22.06.2019 19:10, krisandlance
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1
Do you know the correct answer?
Questions in other subjects:



Mathematics, 26.06.2019 22:00

Mathematics, 26.06.2019 22:00


Mathematics, 26.06.2019 22:00

Mathematics, 26.06.2019 22:00


Mathematics, 26.06.2019 22:00

Mathematics, 26.06.2019 22:00


 , the amount of heat that must be supplied to the substance must be:
, the amount of heat that must be supplied to the substance must be: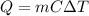
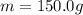 is the mass
is the mass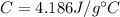 is the specific heat capacity of water
is the specific heat capacity of water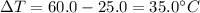 is the increase in temperature
is the increase in temperature