

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 00:30, StayPuftMarshadowMan
What would be the original temperature of a gas that has a volume of 2.0 l and a pressure of 2.0 atm and an unknown temperature that the volume increased to 3.5 l in its pressure decreased to 1.0 atm if the final temperature is measured to be 11°c
Answers: 1


Chemistry, 23.06.2019 16:30, mbonham481
Amodel of an atom is shown below. which element is represented by this model of an atom? boron, carbon, neon, or sodium?
Answers: 1

Chemistry, 23.06.2019 23:30, emwvoidsnake
When the reaction 2h2s(g) 2h2(g) + s2(g) is carried out at 1065°c, kp = 0.012. starting with pure h2s at 1065°, what must the initial pressure of h2s be if the equilibrated mixture at this temperature is to contain 0.250 atm of h2(g)?
Answers: 1
Do you know the correct answer?
The pH at 25 °C of an aqueous solution of the sodium salt of p-monochlorophenol (NaC6H4ClO) is 11.05...
Questions in other subjects:


Chemistry, 17.09.2019 05:00



English, 17.09.2019 05:00




Mathematics, 17.09.2019 05:00

Social Studies, 17.09.2019 05:00


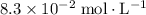 .
.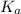 in this question refers the dissociation equilibrium of
in this question refers the dissociation equilibrium of 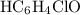 as an acid:
as an acid: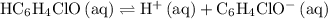 .
. ![\displaystyle K_a\left(\mathrm{HC_6H_4ClO}\right) = \frac{\left[\mathrm{H^{+}}\right] \cdot \left[\mathrm{C_6H_4ClO^{-}}\right]}{\left[\mathrm{HC_6H_4ClO}\right]}](/tpl/images/0619/7247/8ffd8.png) .
.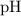 of
of 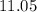 , which means that this solution is basic. In basic solutions at
, which means that this solution is basic. In basic solutions at 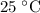 , the concentration of
, the concentration of 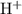 ions is considerably small (typically less than
ions is considerably small (typically less than 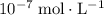 .) Therefore, it is likely not very appropriate to use an equilibrium involving the concentration of
.) Therefore, it is likely not very appropriate to use an equilibrium involving the concentration of 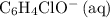 is the conjugate base of the weak acid
is the conjugate base of the weak acid 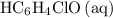 . Therefore, when
. Therefore, when 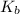 would be equal to
would be equal to 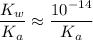 . (
. (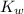 is the self-ionization constant of water.
is the self-ionization constant of water. 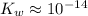 at
at 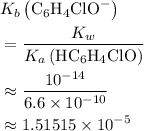 .
.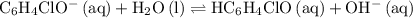 .
.![\displaystyle K_b\left(\mathrm{C_6H_4ClO^{-}}\right) = \frac{\left[\mathrm{HC_6H_4ClO}\right]\cdot \left[\mathrm{OH^{-}}\right]}{\left[\mathrm{C_6H_4ClO^{-}}\right]}](/tpl/images/0619/7247/982ba.png) .
.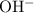 concentration of this solution can be found from its
concentration of this solution can be found from its ![\begin{aligned}& \left[\mathrm{OH^{-}}\right] \\ &= \frac{K_w}{\left[\mathrm{H}^{+}\right]} \\ & = \frac{K_w}{10^{-\mathrm{pH}}} \\ &\approx \frac{10^{-14}}{10^{-11.05}} \\ &\approx 1.1220 \times 10^{-3}\; \rm mol\cdot L^{-1} \end{aligned}](/tpl/images/0619/7247/8b5c5.png) .
.![\left[\mathrm{HC_6H_4ClO}\right]](/tpl/images/0619/7247/a50f4.png) , consider the following table:
, consider the following table: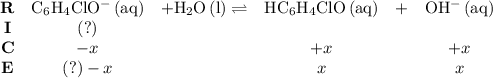
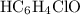 and
and ![\left[\mathrm{HC_6H_4ClO}\right] \approx \left[\mathrm{OH^{-}}\right] \approx 1.1220 \times 10^{-3}\; \rm mol\cdot L^{-1}](/tpl/images/0619/7247/7c60d.png) .
.![\left[\mathrm{C_6H_4ClO^{-}}\right]](/tpl/images/0619/7247/1ccf2.png) from
from 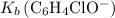 :
:![\begin{aligned} & \left[\mathrm{C_6H_4ClO^{-}}\right] \\ &= \frac{\left[\mathrm{HC_6H_4ClO}\right]\cdot \left[\mathrm{OH^{-}}\right]}{K_b}\\&\approx \frac{\left(1.1220 \times 10^{-3}\right) \times \left(1.1220 \times 10^{-3}\right)}{1.51515\times 10^{-5}}\; \rm mol \cdot L^{-1} \\ &\approx 8.3 \times 10^{-2}\; \rm mol \cdot L^{-1}\end{aligned}](/tpl/images/0619/7247/02e65.png) .
.



