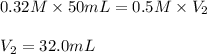The reaction of hydrochloric acid (HCl) with ammonia (NH3) is described by the equation:
HCl + NH3 → NH4Cl
A student is titrating 50 mL of 0.32 M NH3 with 0.5 M HCl. How much hydrochloric acid must be added to react completely with the ammonia?
A. 6.4 mL
B. 16.0 mL
C. 32.0 mL
D. 50.0 mL

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:50, mrylenastewart
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 06:30, dpchill5232
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2
Do you know the correct answer?
The reaction of hydrochloric acid (HCl) with ammonia (NH3) is described by the equation:
...
...
Questions in other subjects:

Mathematics, 12.11.2020 22:30

Mathematics, 12.11.2020 22:30

Mathematics, 12.11.2020 22:30

Mathematics, 12.11.2020 22:30

Mathematics, 12.11.2020 22:30



Health, 12.11.2020 22:30




 are the initial molarity and volume of NH₃.
are the initial molarity and volume of NH₃. are the final molarity and volume of HCl.
are the final molarity and volume of HCl.