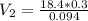Chemistry, 22.04.2020 19:52, blink182lovgabbie
During your reaction, you added 0.3 mL concentrated H2SO4 (18.4 M) as the catalyst. At the end of the reaction, you need to add base to neutralize it. How much volume (in mL) of 10% Na2CO3 to neutralize all the acid present

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, darg3990rgp2t0r2
Does anyone know a lot about how to: - calculate mass of magnesium metal - calculate the actual yield of magnesium oxide - calculate the theoretical yield of mgo - calculate the percent yield of mgo - determine the percent yield of mgo - determine the average percent yield of mgo i had to do an online lab and its asking these questions but i have no idea where to start or how to be able to find these things. i can post the chart of the data from the lab or if you can tell me exactly how i can find each.
Answers: 3

Chemistry, 22.06.2019 00:30, megaaan214p61pb7
Which compounds have the empirical formula ch2o? a. c2h4o2 b. c3h6o3 c. ch2o2 d. c5h10o5 e. c6h12o6
Answers: 3

Chemistry, 22.06.2019 03:00, annafellows
Match term definition ellipse a) diagonal cross section of a cylinder circle b) diagonal cross section through the widest part of a sphere sphere c) cross section parallel to the base of a cone great circle d) shape created when a semi-circle is rotated around the y-axis triangle e) perpendicular cross section of a cone
Answers: 1

Chemistry, 22.06.2019 14:20, greenbyron88
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1
Do you know the correct answer?
During your reaction, you added 0.3 mL concentrated H2SO4 (18.4 M) as the catalyst. At the end of th...
Questions in other subjects:


Mathematics, 25.09.2021 14:00


Mathematics, 25.09.2021 14:00

Social Studies, 25.09.2021 14:00

English, 25.09.2021 14:00

Mathematics, 25.09.2021 14:00


Mathematics, 25.09.2021 14:00