Chemistry, 22.04.2020 20:00, taralynnn8870
When a 0.031M aqueous solution of a certain acid is prepared, the acid is 0.89% dissociated. Calculate the acid dissociation constant Ka of the acid. Round your answer to 2 significant digits.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:00, PinkDivaGirl02
Compare these two waves : a. the blue wave has a higher pitch, but the orange wave is louder. b. the blue and orange waves have the same volume, but the blue wave has a higher pitch. c. the blue and orange waves have the same pitch, but the blue wave is louder. d. the orange wave has a higher pitch, but the blue wave is louder.
Answers: 1

Chemistry, 22.06.2019 10:10, babyphoraaaaa
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate, m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3


Chemistry, 22.06.2019 18:30, bibiansolis
The table lists the lattice energies of some compounds. compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf. the lattice energy increases as the cations get larger, as shown by lif and licl. the lattice energy decreases as cations get smaller, as shown by nacl and naf. the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3
Do you know the correct answer?
When a 0.031M aqueous solution of a certain acid is prepared, the acid is 0.89% dissociated. Calcula...
Questions in other subjects:

Mathematics, 29.10.2020 20:40


Chemistry, 29.10.2020 20:40

Mathematics, 29.10.2020 20:40



Mathematics, 29.10.2020 20:40

Mathematics, 29.10.2020 20:40




 due to the reaction extent, the percent dissociation is:
due to the reaction extent, the percent dissociation is:![\% Dissociation:\frac{x}{[acid]_0}](/tpl/images/0618/9112/63f66.png)
![x=\% Dissociation*[acid]_0=0.89\%*0.031M=2.759x10^{-4}M](/tpl/images/0618/9112/fb10b.png)
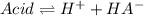
![Ka=\frac{x*x}{[acid]_0-x}=\frac{2.759x10^{-4}M*2.759x10^{-4}M}{0.031M-2.759x10^{-4}M} \\\\Ka=2.5x10^{-6}](/tpl/images/0618/9112/d4830.png)