
Chemistry, 22.04.2020 19:00, arieannaensley0616
Given the balanced equation 2C4H10 + 13O2 → 8CO2 + 10H2O, how many moles of CO2 are produced when 14.9g of O2 are used?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, rigobertogarza2
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2


Chemistry, 22.06.2019 17:00, brandiwingard
What is the mass of phosphorous in a 51-kg person
Answers: 1

Chemistry, 22.06.2019 19:00, HaydenSturgis1
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3
Do you know the correct answer?
Given the balanced equation 2C4H10 + 13O2 → 8CO2 + 10H2O, how many moles of CO2 are produced when 14...
Questions in other subjects:

Mathematics, 22.02.2021 03:00


History, 22.02.2021 03:00

Arts, 22.02.2021 03:00

Mathematics, 22.02.2021 03:00

Biology, 22.02.2021 03:00


Mathematics, 22.02.2021 03:00

Mathematics, 22.02.2021 03:00

Mathematics, 22.02.2021 03:00


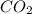 produced are, 0.287 moles.
produced are, 0.287 moles.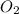 = 14.9 g
= 14.9 g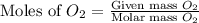
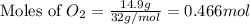
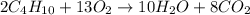
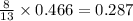 mole of
mole of 



