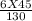Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:50, adrian08022
Use the standard enthalpies of formation for the reactants and products to solve for the δhrxn for the following reaction. (the δhf of c2h4 is 52.26 kj/mol, co2 is -393.509 kj/mol, and h2o is -241.818 kj.) c2h4 (g) + 3o2(g) 2co2 (g) + 2h2o(g) δhrxn = the reaction is .
Answers: 3

Chemistry, 22.06.2019 04:30, clairajogriggsk
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1

Chemistry, 22.06.2019 12:40, valenzueladomipay09u
How does concentration affect reaction rate
Answers: 2

Chemistry, 23.06.2019 01:00, daniel1480
Which fossil fuel is mainly used for heating and cooking? a. electricity b. coal c. petroleum d. natural gas
Answers: 2
Do you know the correct answer?
If 45.0 mL of 6.00M HCl is added to 130 mL of H2O, Determine the molarity of the final solution. (sh...
Questions in other subjects:

Mathematics, 11.03.2021 07:20

Mathematics, 11.03.2021 07:20





Mathematics, 11.03.2021 07:20

Mathematics, 11.03.2021 07:20

Mathematics, 11.03.2021 07:20

History, 11.03.2021 07:20


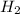 O.
O.