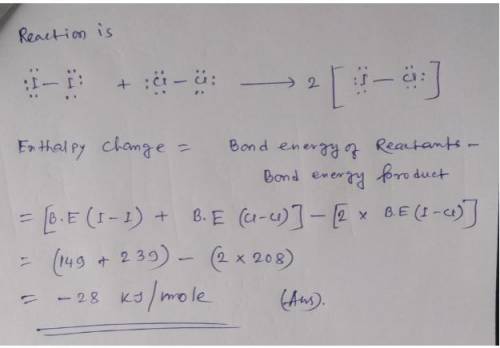Chemistry, 22.04.2020 04:27, tobyhollingsworth178
Calculate enthalpy of reaction using bond energies. Use the References to access important values if needed for this question. Use average bond enthalpies (linked above) to calculate the enthalpy change for the following gas-phase reaction. I2(g) +Cl2(g) 2ICI(g) To analyze the reaction, first draw Lewis structures for all reactant and product molecules. Include all valence lone pairs in your answer. Draw the reaction using separate sketchers for each species. One molecule per sketcher, please. Separate multiple reactants and/or products using the + sign from the drop-down arrow. Separate reactants from products using the symbol from the drop-down menu. If you have to draw H2, draw H-H.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:50, AysiaRamosLee
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 10:30, kylemartinez13
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 22.06.2019 10:50, adam1299
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments, solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1

Chemistry, 22.06.2019 12:00, 1963038660
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1
Do you know the correct answer?
Calculate enthalpy of reaction using bond energies. Use the References to access important values if...
Questions in other subjects:

Mathematics, 15.05.2021 01:40

Mathematics, 15.05.2021 01:40






Biology, 15.05.2021 01:40