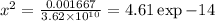Chemistry, 22.04.2020 04:27, laylay7383
G Using the complex based titration system: 50.00 mL 0.00250 M Ca2 titrated with 0.0050 M EDTA, buffered at pH 11.0 determine (i) first pCa first before initiating the titration process and then (ii) at equivalence when all the Ca2 is titrated to CaY2-. Please, use your text books and/or lecture notes to find potentially missing information about constants needed to solve the problem.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, mayamabjishovrvq9
1. calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has the same thermal mass as 1.0 ml of water. iqnore the thermal mass of th sodium bicarbonate. note: it takes about 4.2 joules () to change 1.0 gram (1.0ml) of water 1.0 c
Answers: 2


Do you know the correct answer?
G Using the complex based titration system: 50.00 mL 0.00250 M Ca2 titrated with 0.0050 M EDTA, buff...
Questions in other subjects:

Mathematics, 04.02.2021 16:40



Biology, 04.02.2021 16:40

History, 04.02.2021 16:40


Biology, 04.02.2021 16:40


English, 04.02.2021 16:40

Mathematics, 04.02.2021 16:40


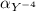 =0.81
=0.81 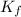 =0.81x 4.47 x10¹⁰
=0.81x 4.47 x10¹⁰![[CaY^{2-}] = \frac{Initial,moles,of, Ca^{2+}}{Total,Volume} = \frac{0.125mol}{(50.00+25.00)mL} = 0.001667M](/tpl/images/0617/6168/49a70.png)
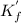
![{K^'}_f = \frac{[CaY^{2-}]}{[Ca^{2+}][Y^4]}=\frac{0.001667-x}{x.x} =\frac{0.001667-x}{x^2}\\\\x^2 = \frac{0.001667-x}{{K^'}_f}\\ \\](/tpl/images/0617/6168/d054e.png)