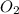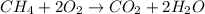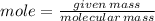13) Methane (CH4) combines with oxygen according to the balanced equation below. What is the limiting reactant if 25.0 g of CH4 combines with 50.0 g of O2? CH4 + 2 O2 → CO2 + 2 H2O moles CH4 moles O2 moles O2 needed to react with given amount of CH4 limiting reactant ("methane" or "oxygen")

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, phebusadrian01
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 23.06.2019 01:00, tjeffers90028
Iron (fe) reacts with copper sulfate (cuso4) to form iron (ii) sulfate. in this reaction, cu2+ gains electrons to form cu. which statement is true about this reaction? fe(s) + cuso4(aq) → feso4(aq) + cu(s)
Answers: 3

Chemistry, 23.06.2019 01:00, breemills9552
What two factors can affect the properties of a hydrocarbon? a. the number of its carbon atoms and the number of single bonds b. the number of its carbon atoms and the arrangement of its atoms c. the arrangement of its atoms and the number of its double bonds
Answers: 1

Chemistry, 23.06.2019 12:30, marleacorey
All chemicals, even safe chemicals, such as sodium chloride( table salt), are toxic if exposure is high enough. (true or false)
Answers: 2
Do you know the correct answer?
13) Methane (CH4) combines with oxygen according to the balanced equation below. What is the limitin...
Questions in other subjects:


Health, 31.08.2019 21:10

Mathematics, 31.08.2019 21:10


Mathematics, 31.08.2019 21:10


English, 31.08.2019 21:10

Mathematics, 31.08.2019 21:10

Mathematics, 31.08.2019 21:10