
Chemistry, 22.04.2020 02:08, alexis9263
If a solute dissolves in an endothermic process Question 10 options: a) the solute must be a gas. b) the entropy of the solution must be greater than that of its pure components. c) H bonds must exist between solvent and solute. d) the entropy of the solution is immaterial. e) strong ion-dipole forces must exist in the solution.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, waterborn7152
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2


Chemistry, 22.06.2019 20:30, Schoolworkspace453
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2

Chemistry, 23.06.2019 09:30, raymondmancilla123
The mass of a proton is approximately equal to the mass of
Answers: 1
Do you know the correct answer?
If a solute dissolves in an endothermic process Question 10 options: a) the solute must be a gas. b)...
Questions in other subjects:

Social Studies, 08.07.2019 12:00

World Languages, 08.07.2019 12:00


Advanced Placement (AP), 08.07.2019 12:00

Mathematics, 08.07.2019 12:00

History, 08.07.2019 12:00

Mathematics, 08.07.2019 12:00

Mathematics, 08.07.2019 12:00



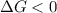 .
. .......... (1)
.......... (1) (for an endothermic process).
(for an endothermic process).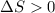 ) more will be the negative value of
) more will be the negative value of  .
.




