
Chemistry, 22.04.2020 01:56, julielebo8
The free-energy change of a reaction is a measure of a. the excess entropy given off to the reaction system. b. the increased molecular disorder that occurs in the system. c. the energy given off to the surroundings. d. the direction in which a net reaction occurs. e. the excess entropy given off to the surroundings

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:30, ashleybarrera2000
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3


Chemistry, 22.06.2019 16:00, ghadeeraljelawy
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2
Do you know the correct answer?
The free-energy change of a reaction is a measure of a. the excess entropy given off to the reaction...
Questions in other subjects:


Mathematics, 24.02.2022 14:00




Mathematics, 24.02.2022 14:00

SAT, 24.02.2022 14:00



Mathematics, 24.02.2022 14:00


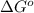 . Free-energy helps in determining the direction of a chemical reaction like if it is taking place in forward or backward reaction.
. Free-energy helps in determining the direction of a chemical reaction like if it is taking place in forward or backward reaction.



