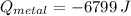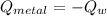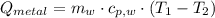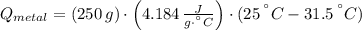Chemistry, 22.04.2020 02:39, rodolfoperezzz1332
A 20.0 g piece of a metal is heated and place into a calorimeter containing 250.0 g of water initially at 25.0 oC. The final temperature of the water is 31.5 oC. What is the heat change of the metal in joules? The specific heat of water is 4.184 J/goC

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:40, jaueuxsn
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2

Chemistry, 22.06.2019 12:00, WinterStrikesBack
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 13:00, monkeyrose1999
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3
Do you know the correct answer?
A 20.0 g piece of a metal is heated and place into a calorimeter containing 250.0 g of water initial...
Questions in other subjects:

Computers and Technology, 12.02.2022 14:00




Spanish, 12.02.2022 14:00



English, 12.02.2022 14:00

Biology, 12.02.2022 14:00