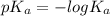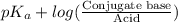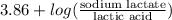"" What is the pH of a solution that is prepared by dissolving 8.52 grams of lactic acid (formula weight = 90.08 grams/mol) and 7.93 grams of sodium lactate (formula weight = 112.06 grams/mole) in water and diluting to 500.00 mL? The Ka for lactic acid is 0.000137.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:40, whitethunder05
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2

Chemistry, 22.06.2019 13:30, justinerodriguz2878
What are the major types of a chemical compound
Answers: 2

Chemistry, 22.06.2019 17:00, destinyycooper
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2
Do you know the correct answer?
"" What is the pH of a solution that is prepared by dissolving 8.52 grams of lactic acid (formula we...
Questions in other subjects:

Chemistry, 02.04.2020 16:26

Mathematics, 02.04.2020 16:26


History, 02.04.2020 16:27



Mathematics, 02.04.2020 16:27


Physics, 02.04.2020 16:27


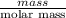
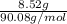
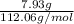
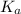 and
and  is as follows.
is as follows.