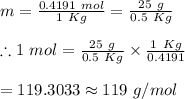Chemistry, 22.04.2020 01:46, slowik9467
25 g of a compound is added to 500 mL of water if the freezing point of the resulting solution is
0.57 °C what is the molecular weight of the compound assume no molecular disassociation upon
dissolution Kf equals 1.36 °C/m
O 119 g/mol
90 g/mol
0 60 g/mol
238 g/mol

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, Falconpride4079
Construct the hypothetical phase diagram for metals a and b between room temperature (20c) and 700c, given the following information: * the melting temperature of metal a is 480c. • the maximum solubility of b in a is 4 wt% b, which occurs at 420c. • the solubility of b in a at room temperature is 0 wt% b. • one eutectic occurs at 420c and 18 wt% b–82 wt% a. • a second eutectic occurs at 475c and 42 wt% b–58 wt% a. • the intermetallic compound ab exists at a composition of 30 wt% b–70 wt% a, and melts congruently at 525c.• the melting temperature of metal b is 600c. • the maximum solubility of a in b is 13 wt% a, which occurs at 475c. • the solubility of a in b at room temperature is 3 wt% a.
Answers: 1

Chemistry, 22.06.2019 05:30, sethjohnson386pbnm3x
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3


Chemistry, 23.06.2019 03:00, kuehlthau03
Describe the properties of sodium, chlorine, and sodium chloride
Answers: 1
Do you know the correct answer?
25 g of a compound is added to 500 mL of water if the freezing point of the resulting solution is
Questions in other subjects:


Mathematics, 25.03.2020 21:37



History, 25.03.2020 21:37


Chemistry, 25.03.2020 21:37