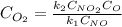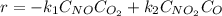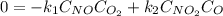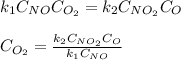Onsider the following elementary reaction:
NO_(g) +O_2(g) → NO_2(g) + O_(g)
Suppose we...

Chemistry, 22.04.2020 00:33, rbalexander25
Onsider the following elementary reaction:
NO_(g) +O_2(g) → NO_2(g) + O_(g)
Suppose we let k_1 stand for the rate constant of this reaction, and k_-1 stand for the rate constant of the reverse reaction. Write an expression that gives the equilibrium concentration of O_2 in terms of k_1 k_-1. and the equilibrium concentrations of NO NO_2 and O. [O_2] =

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, ayoismeisalex
Matches the chemical name of each oxide of phosphorus to its chemical formula
Answers: 2


Chemistry, 22.06.2019 06:30, yolo123321
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 22.06.2019 07:20, JKINGblackstar3502
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1
Do you know the correct answer?
Questions in other subjects:









English, 08.10.2019 23:30