
Chemistry, 22.04.2020 00:20, shyshy6184
It is proposed to use Liquid Petroleum Gas (LPG) to fuel spark-ignition engines. A typical sample of the fuel on a volume basis consists of: 70% propane C3H8; 5% butane C4H10; and 25% propene C3H6 The higher heating values of the fuels are 50.38 MJ/kg for propane, 49.56 MJ/kg for butane, and 48.95 MJ/kg for propene. a) Work out the overall combustion reaction for stoichiometric combustion of 1 mole of LPG with air, and determine the stoichiometric F/A and A/F ratios. b) What are the higher and lower heating values per unit mass of LPG?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, lpssprinklezlps
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3


Do you know the correct answer?
It is proposed to use Liquid Petroleum Gas (LPG) to fuel spark-ignition engines. A typical sample of...
Questions in other subjects:

Mathematics, 22.10.2020 02:01


Mathematics, 22.10.2020 02:01



Biology, 22.10.2020 02:01

Physics, 22.10.2020 02:01



Mathematics, 22.10.2020 02:01



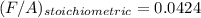
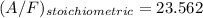
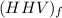 per unit mass of LPG = 49.9876 MJ/kg
per unit mass of LPG = 49.9876 MJ/kg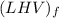 per unit mass of LPG = 46.4933 MJ/kg
per unit mass of LPG = 46.4933 MJ/kg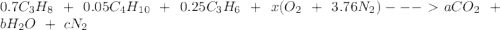
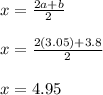
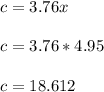
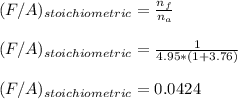
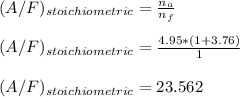
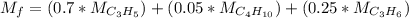

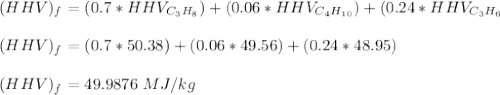
![(LHV)_f = (HHV)_f - \delta H_w \\ \\ (LHV)_f = (HHV)_f - [\frac{m_w}{m_f}h_{vap}] \\ \\ (LHV)_f = 49.9876 \ MJ/kg - [\frac{3.8*18}{44.2}*2.258 \ MJ/kg] \\ \\ (LHV)_f = 46.4933 \ M/kg](/tpl/images/0616/4836/e9631.png)




