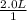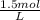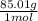Chemistry, 22.04.2020 00:20, venuscagle
1. What mass of the following chemicals is needed to make the solutions indicated?
a. 1.0 liter of a 1.0 M mercury (II) chloride (HgCl2) solution
b. 2.0 liters of a 1.5 M sodium nitrate (NaNO3) solution
c. 5.0 liters of a 0.1 M Ca(OH)2 solution
d. 100 mL of a 0.5 M (NH4)3PO4 solution
2. Calculate the molarity of the following solutions.
a. 12 g of lithium hydroxide (LiOH) in 1.0 L of solution
b. 198 g of barium bromide (BaBr2) in 2.0 L of solution
c. 54 g of calcium sulfide (CaS) in 3.0 L of solution
3. Calculate the volume of each solution, in liters.
a. a 1.0 M solution containing 85 g of silver nitrate (AgNO3)
b. a 0.5 M solution containing 250 g of manganese (II) chloride (MnCl2)
c. a 0.4 M solution containing 290 g of aluminum nitrate (Al(NO3)3)

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 23.06.2019 01:30, jarteria0
Some molecular compounds, such as hcl, ionize completely in solution. for molecular compounds such as h2co3, most molecules do not ionize in solution. which describes the properties of these two solutes? a. hcl and h2co3 have the same effect on the properties of the solution. b. hcl raises the freezing point of water more than h2co3 does. c. hcl raises the boiling point of water more than h2co3 does.
Answers: 2

Chemistry, 23.06.2019 05:00, MoneyMike42
Select the statement that describe chemical properties a. antacid tablets neutralize stomach acid b. helium is the lightest monatomic element c. water freezes at 0 celsius d. mercury is liquid at room temperature
Answers: 3
Do you know the correct answer?
1. What mass of the following chemicals is needed to make the solutions indicated?
a. 1....
a. 1....
Questions in other subjects:

Mathematics, 08.06.2021 02:40

Social Studies, 08.06.2021 02:40

Social Studies, 08.06.2021 02:40

SAT, 08.06.2021 02:40

Mathematics, 08.06.2021 02:40

Chemistry, 08.06.2021 02:40


Mathematics, 08.06.2021 02:40

Mathematics, 08.06.2021 02:40