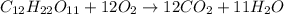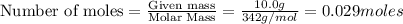Chemistry, 21.04.2020 23:31, Sillydork4545
The equation represents the combustion of sucrose. C12H22O11 + 12O2 Right arrow. 12CO2 + 11H2O If there are 10.0 g of sucrose and 8.0 g of oxygen, how many moles of sucrose are available for this reaction? 0.029 mol 0.250 mol 0.351 mol 3.00 mol

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, Falconpride4079
Construct the hypothetical phase diagram for metals a and b between room temperature (20c) and 700c, given the following information: * the melting temperature of metal a is 480c. • the maximum solubility of b in a is 4 wt% b, which occurs at 420c. • the solubility of b in a at room temperature is 0 wt% b. • one eutectic occurs at 420c and 18 wt% b–82 wt% a. • a second eutectic occurs at 475c and 42 wt% b–58 wt% a. • the intermetallic compound ab exists at a composition of 30 wt% b–70 wt% a, and melts congruently at 525c.• the melting temperature of metal b is 600c. • the maximum solubility of a in b is 13 wt% a, which occurs at 475c. • the solubility of a in b at room temperature is 3 wt% a.
Answers: 1


Chemistry, 22.06.2019 20:30, sydneip6174
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2

Chemistry, 23.06.2019 01:00, skatelife8974
What is the chemical name of the compound ti2o3?
Answers: 2
Do you know the correct answer?
The equation represents the combustion of sucrose. C12H22O11 + 12O2 Right arrow. 12CO2 + 11H2O If th...
Questions in other subjects:





Mathematics, 26.02.2020 21:30






 of particles.
of particles.