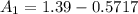Chemistry, 21.04.2020 22:35, tiniecisneros28
A concentration cell based on the following half reaction at 289 K has initial concentrations of 1.39 M , 0.312 M , and a potential of 0.037206 V at these conditions. After 9.1 hours, the new potential of the cell is found to be 0.0095376 V. What is the concentration of at the cathode at this new potential

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, mildredelizam
Select the correct answer. you have a nightlight plugged into an outlet in the hallway, which uses 3.5 watts when plugged in. if the house circuit provides 120.0 volts, what is the current through this bulb?
Answers: 1


Chemistry, 22.06.2019 00:00, tahjaybenloss16
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 04:00, heavyhearttim
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2
Do you know the correct answer?
A concentration cell based on the following half reaction at 289 K has initial concentrations of 1.3...
Questions in other subjects:



Mathematics, 12.02.2021 06:10






Mathematics, 12.02.2021 06:10


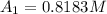
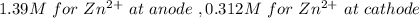
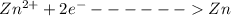
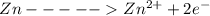
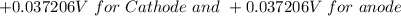
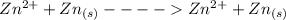
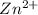 at cathode loss some concentration and
at cathode loss some concentration and 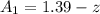
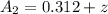
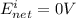
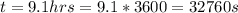
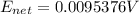
![E_{cell} = E^i_{cell}- \frac{RT}{nF} ln[\frac{[A_2]}{[A_2]} ]](/tpl/images/0616/0641/f278f.png)
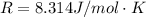
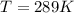
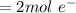
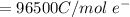
![0.0095376 =0.0 -\frac{[8.314][288]}{[2] [9600]} ln [\frac{(0.312 +z)}{(1.39 -z)} ]](/tpl/images/0616/0641/c9c95.png)
![0.0095376 = 0.12406383 \ ln [\frac{( 0.312 + z)}{[1.39 -z]} ]](/tpl/images/0616/0641/96214.png)
![\frac{0.0095376 }{ 0.12406383} = \ ln [\frac{( 0.312 + z)}{[1.39 -z]} ]](/tpl/images/0616/0641/52f0e.png)
![0.0768766 = \ ln [\frac{( 0.312 + z)}{[1.39 -z]} ]](/tpl/images/0616/0641/3085d.png)
![e^{0.0768766 }= [\frac{( 0.312 + z)}{[1.39 -z]} ]](/tpl/images/0616/0641/42989.png)
![1.0799 = [\frac{( 0.312 + z)}{[1.39 -z]} ]](/tpl/images/0616/0641/39ed8.png)
![[1.39 -z ]1.0799 = 0.312 + z \\\\1.501 - 1.0799z = 0.312 +z\\\\1.501-0.312 = 1.0799z + z\\\\1.1891 =2.0799z\\\\ z =\frac{1.1891}{2.0799}\\\\ z =0.5717](/tpl/images/0616/0641/109ca.png)