Chemistry, 21.04.2020 20:51, genyjoannerubiera
A gas in an engine cylinder expands from a volume of 10.0 L to 15.0 L against an external pressure of 1 atm and the system absorbs 300 J of heat in the process. Determine the work done by the system and the change in the system's internal energy, both in joules. Use this conversion scale to calculate the work done in joules: 1 L * atm

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, dice50
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1

Chemistry, 22.06.2019 21:00, estherdinhllama
Read "who built the pyramids? ”. leave this link open while you answer the questions throughout the assignment. give at least two reasons why some people claim the pyramids of giza were constructed by aliens.
Answers: 1


Chemistry, 23.06.2019 01:30, Nathaliasmiles
If a particle has z = 25 and 23 electrons, what is its charge?
Answers: 2
Do you know the correct answer?
A gas in an engine cylinder expands from a volume of 10.0 L to 15.0 L against an external pressure o...
Questions in other subjects:



Mathematics, 06.10.2021 14:00







Mathematics, 06.10.2021 14:00


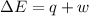
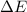 =Change in internal energy
=Change in internal energy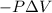 {Work is done by the system as the final volume is greater than initial volume and is negative}
{Work is done by the system as the final volume is greater than initial volume and is negative}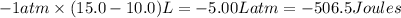 {1Latm=101.3J}
{1Latm=101.3J}