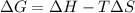Chemistry, 21.04.2020 20:49, pacerskora
G The reaction C(s) + CO2(g) → 2CO(g) is spontaneous only at temperatures in excess of 1100 K. We can conclude that a. ΔG° is negative for all temperatures. b. ΔH° is negative and ΔS° is negative. c. ΔH° is positive and ΔS° is positive. d. ΔH° is negative and ΔS° is positive. e. ΔH° is positive and ΔS° is negative.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:10, lindasuebairdoyjpf7
How many grams of naoh are needed to make 0.250 liter of a 0.500 m solution of naoh? 0.125 g 5.00 g 2.00 g
Answers: 1


Chemistry, 22.06.2019 09:40, cheesecake1919
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 13:30, suemmimonjaras8374
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1
Do you know the correct answer?
G The reaction C(s) + CO2(g) → 2CO(g) is spontaneous only at temperatures in excess of 1100 K. We ca...
Questions in other subjects:




Social Studies, 09.07.2019 17:00






Mathematics, 09.07.2019 17:00