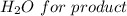Chemistry, 21.04.2020 20:39, allierl2001
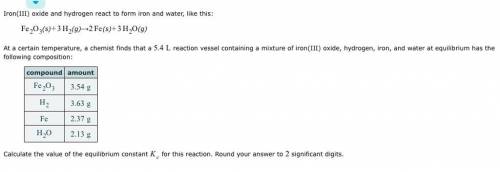
Iron(III) oxide and hydrogen react to form iron and water, like this: Fe_2O_3(s) + 3H_2(g) rightarrow 2Fe(s) + 3H_2O(g) At a certain temperature, a chemist finds that a 5.4 L reaction vessel containing a mixture of iron(III) oxide, hydrogen, iron, and water at equilibrium has the following composition: Calculate the value of the equilibrium constant K_c for this reaction. Round your answer to 2 significant digits.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:10, shafferakr6
There are 6.022 x 10^23 atoms of hg in 1 mole of hg. the number of atoms in 4.5 moles of hg can be found by multiplying 4.5 by 6.022 x 10^23 a. 2.7 x 10^24 b. 27 x 10^23 c. 2.71 x10^24 d. 27.099 x 10^23
Answers: 3

Chemistry, 22.06.2019 10:00, Cythina2007
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 22.06.2019 23:00, maddyleighanne
Arectangle has a diagonal 20 inches long that forms angles of 60 and 30 with the sides. find the perimeter of the rectangle. for geometry
Answers: 3
Do you know the correct answer?
Iron(III) oxide and hydrogen react to form iron and water, like this: Fe_2O_3(s) + 3H_2(g) rightarro...
Questions in other subjects:


Mathematics, 21.12.2020 20:50


Business, 21.12.2020 20:50

Mathematics, 21.12.2020 20:50


Mathematics, 21.12.2020 20:50



Mathematics, 21.12.2020 20:50



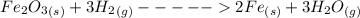
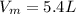
 is a constant with value of
is a constant with value of 
 is a constant with value of
is a constant with value of 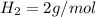
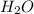 is a constant with value of
is a constant with value of 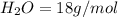
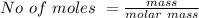
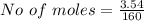
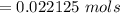


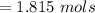
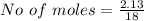
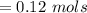
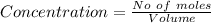
![Concentration[Fe_2 O_3] = \frac{0.222125}{5.4}](/tpl/images/0615/6667/c3dcc.png)
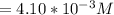
![Concentration[H_2] = \frac{1.815}{5.4}](/tpl/images/0615/6667/26a72.png)
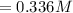
![Concentration [H_2O] = \frac{0.12}{5.4}](/tpl/images/0615/6667/14bc0.png)
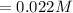
![K_c = \frac{[concentration \ of \ product]}{[concentration \ of \ reactant ]}](/tpl/images/0615/6667/26746.png)