
Chemistry, 21.04.2020 20:48, 25jzaldivar
The decomposition reaction 2 NOCl → 2 NO + Cl_2 has a rate law that is second order with respect to [NOCl], where k = 3.2 M^{-1}s^{-1} at a certain temperature. If the initial concentration of NOCl is 0.076 M, how many seconds will it take for [NOCl] to decrease to 0.042 M at this temperature? Do not enter units with your numerical answer.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:20, mathman783
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1



Chemistry, 23.06.2019 03:00, rayne40
The size (radius) of an oxygen molecule is about 2.0 ×10−10m. make a rough estimate of the pressure at which the finite volume of the molecules should cause noticeable deviations from ideal-gas behavior at ordinary temperatures (t= 300k ). assume that deviatons would be noticeable when volume of the gas per molecule equals the volume of the molecule itself.
Answers: 3
Do you know the correct answer?
The decomposition reaction 2 NOCl → 2 NO + Cl_2 has a rate law that is second order with respect to...
Questions in other subjects:





Mathematics, 23.03.2020 16:50


Mathematics, 23.03.2020 16:50


English, 23.03.2020 16:50


![\frac{1}{[NOCl]}=kt+\frac{1}{[NOCl]_{0}}](/tpl/images/0615/7124/81c05.png)
![[NOCl]_{0}](/tpl/images/0615/7124/069d3.png) is initial concentration of NOCl and k is rate constant.
is initial concentration of NOCl and k is rate constant.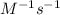 and [NOCl] = 0.042 M
and [NOCl] = 0.042 M![\frac{1}{0.042M}=[3.2M^{-1}s^{-1}\times t]+\frac{1}{0.076M}](/tpl/images/0615/7124/ee865.png)




