Chemistry, 21.04.2020 18:20, Isabella1319
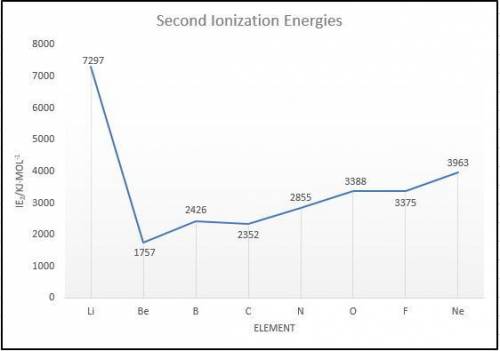
Elements in group 7A in the periodic table are called the halogens: elements in group 6A are called the chalcogens (a)
What is the most common oxidation state of the chalcogens compared to the halogens? (b) For each of the following
periodic properties. state whether the halogens or the chalcogens have larger values: atomic radii, ionic radii of the most
common oxidation state, first ionization energy. second ionization energy.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:10, tishfaco5000
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2

Chemistry, 22.06.2019 08:00, danielhall
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1

Chemistry, 22.06.2019 13:30, kassandrarosario1115
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1
Do you know the correct answer?
Elements in group 7A in the periodic table are called the halogens: elements in group 6A are called...
Questions in other subjects:


Mathematics, 18.10.2020 16:01

Mathematics, 18.10.2020 16:01



Mathematics, 18.10.2020 16:01

Mathematics, 18.10.2020 16:01



Mathematics, 18.10.2020 16:01