
Chlorine can be prepared in the laboratory by the reaction of manganese dioxide with hydrochloric acid, HCl(aq) , as described by the chemical equation MnO2(s)+4HCl(aq)⟶MnCl2(aq)+2H2O(l)+ Cl2(g) How much MnO2(s) should be added to excess HCl(aq) to obtain 205 mL Cl2(g) at 25 °C and 705 Torr ?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, bryneosburn
What’s a special glass that most beakers are made of
Answers: 1


Chemistry, 22.06.2019 08:30, waterborn7152
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2
Do you know the correct answer?
Chlorine can be prepared in the laboratory by the reaction of manganese dioxide with hydrochloric ac...
Questions in other subjects:



Arts, 06.10.2019 19:00

History, 06.10.2019 19:00


Chemistry, 06.10.2019 19:00



History, 06.10.2019 19:00


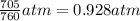
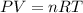 ( ideal gs equation)
( ideal gs equation)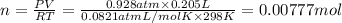
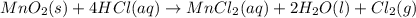
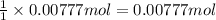 of manganese (IV) oxide
of manganese (IV) oxide



