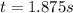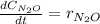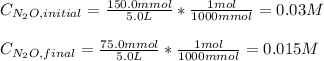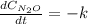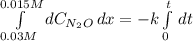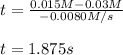Chemistry, 21.04.2020 17:30, amandajbrewerdavis
Under certain conditions the rate of this reaction is zero order in dinitrogen monoxide with a rate constant of ·0.0080Ms−1: 2N2O(g)→2N2(g)+O2(g) Suppose a 5.0L flask is charged under these conditions with 150.mmol of dinitrogen monoxide. After how much time is there only 75.0mmol left? You may assume no other reaction is important.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, Unknowndragon42
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 20:30, jaydenbrock
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1

Chemistry, 22.06.2019 22:40, destineysarah
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1

Chemistry, 23.06.2019 01:00, tjeffers90028
Iron (fe) reacts with copper sulfate (cuso4) to form iron (ii) sulfate. in this reaction, cu2+ gains electrons to form cu. which statement is true about this reaction? fe(s) + cuso4(aq) → feso4(aq) + cu(s)
Answers: 3
Do you know the correct answer?
Under certain conditions the rate of this reaction is zero order in dinitrogen monoxide with a rate...
Questions in other subjects:

History, 01.03.2021 19:30



Mathematics, 01.03.2021 19:30



History, 01.03.2021 19:30


Mathematics, 01.03.2021 19:30