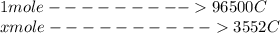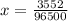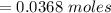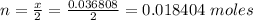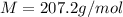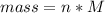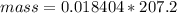Chemistry, 21.04.2020 15:30, sophcent5828
Problem PageQuestion When a lead acid car battery is recharged by the alternator, it acts essentially as an electrolytic cell in which solid lead(II) sulfate is reduced to lead at the cathode and oxidized to solid lead(II) oxide at the anode. Suppose a current of is fed into a car battery for seconds. Calculate the mass of lead deposited on the cathode of the battery. Round your answer to significant digits. Also, be sure your answer contains a unit symbol.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:40, MathChic68
It is important to wear proper protective equipment in lab even when not actively performing experiments because accidents can affect any researcher, even one not working on an experiment. select the best answer from the choices provided
Answers: 3

Chemistry, 22.06.2019 05:00, adjjones2011
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1

Chemistry, 22.06.2019 12:30, skaterwolf1317
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Do you know the correct answer?
Problem PageQuestion When a lead acid car battery is recharged by the alternator, it acts essentiall...
Questions in other subjects:


Chemistry, 08.07.2020 01:01

Biology, 08.07.2020 01:01


Mathematics, 08.07.2020 01:01

Mathematics, 08.07.2020 01:01

History, 08.07.2020 01:01

Biology, 08.07.2020 01:01

Mathematics, 08.07.2020 01:01


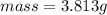
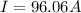
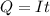
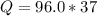
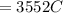
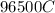
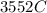 would contain how many moles
would contain how many moles