Chemistry, 21.04.2020 04:12, Riplilpeep
A gas occupies 3.5 L at standard pressure. Find the volume of the gas when the pressure is 1.5 atm. (Remember, standard pressure equals 101.3kPa, 1atm, or 760mmHg.)

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 22:30, darceline1574
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

Chemistry, 23.06.2019 06:00, asalimanoucha2v
•what conclusions can you make about the relationship between the volume of a gas and its temperature? • what conclusions can you make about the relationship between the volume of a gas and its pressure? • what possible variables have you not accounted for? as you did the procedures, is it possible that the atmospheric pressure may have changed? if it did change over the course of your experiment, then how would your results have been affected?
Answers: 3
Do you know the correct answer?
A gas occupies 3.5 L at standard pressure. Find the volume of the gas when the pressure is 1.5 atm....
Questions in other subjects:

Mathematics, 20.01.2021 22:20

Computers and Technology, 20.01.2021 22:20


Biology, 20.01.2021 22:20

Mathematics, 20.01.2021 22:20


Mathematics, 20.01.2021 22:20

Mathematics, 20.01.2021 22:20

Mathematics, 20.01.2021 22:20

Computers and Technology, 20.01.2021 22:20


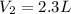
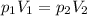
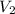 , we obtain:
, we obtain: