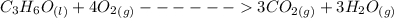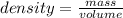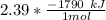Consider the thermochemical equation for the combustion of acetone (c3h6o), the main ingredient in nail polish remover. c3h6o (l) + 4 o2 (g) à 3 co2 (g) + 3 h2o (g) ∆horxn = -1790 kj if a bottle of nail polish remover contains 177 ml of acetone, how much heat is released by its complete combustion? the density of acetone is 0.788 g/ml.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, anamaliiow
Acylinder is filled with 2.00 moles of nitrogen, 3.00 moles of argon and 5.00 moles of helium. if the gas mixture is at stp, what is the partial pressure of the argon
Answers: 1

Chemistry, 22.06.2019 04:00, eborkins
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3


Chemistry, 22.06.2019 16:00, yfnal3x
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2
Do you know the correct answer?
Consider the thermochemical equation for the combustion of acetone (c3h6o), the main ingredient in n...
Questions in other subjects:






Business, 17.07.2019 04:30

Mathematics, 17.07.2019 04:30

English, 17.07.2019 04:30

Social Studies, 17.07.2019 04:30